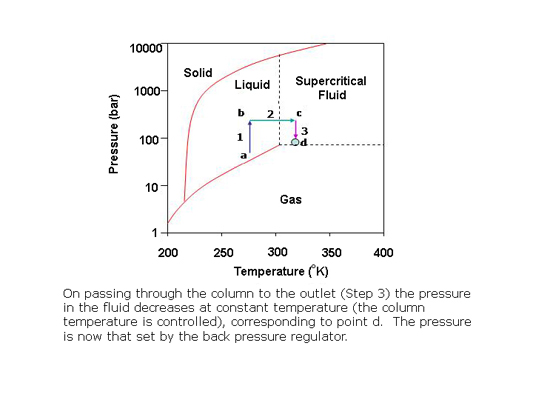
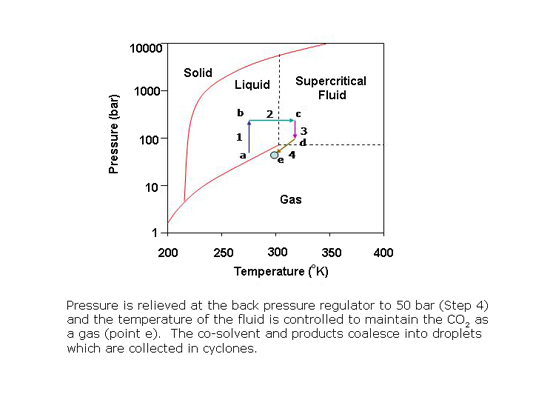
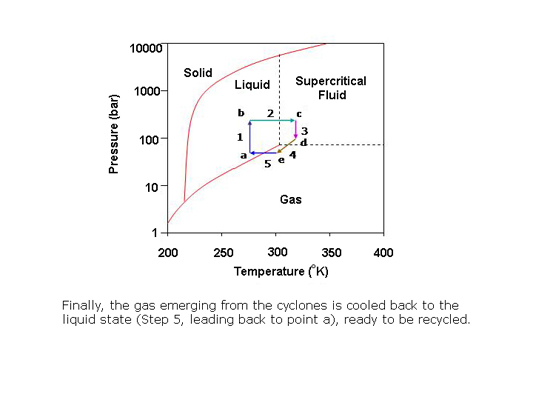
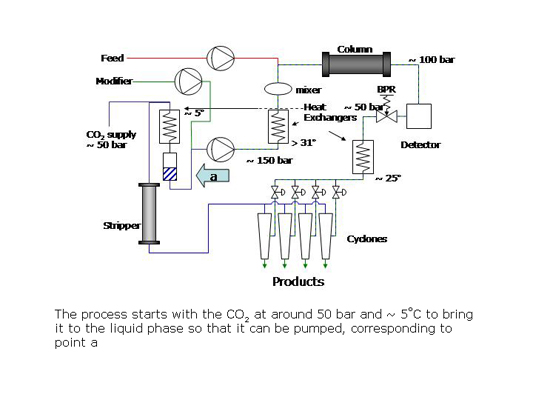
What is a supercritical fluid?
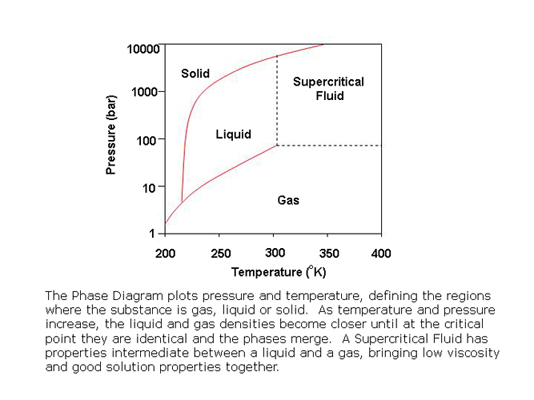
A supercritical fluid is one that has been heated and compressed sufficiently that the vapor and liquid phases have the same density and cannot be distinguished. The temperature and pressure at which this occurs is called the critical point. The critical point for carbon dioxide is conveniently attainable, allowing its use as a chromatographic mobile phase.
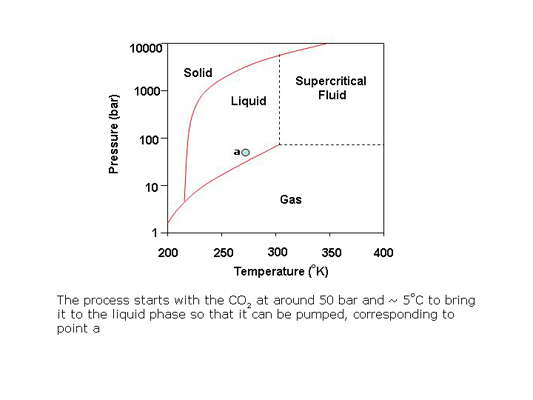
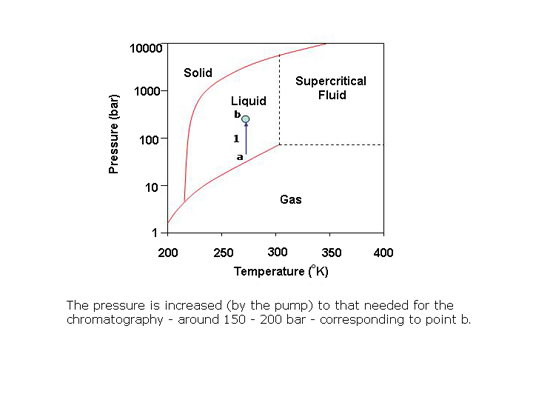
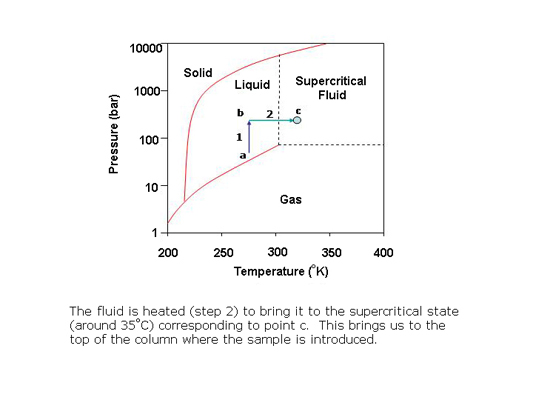
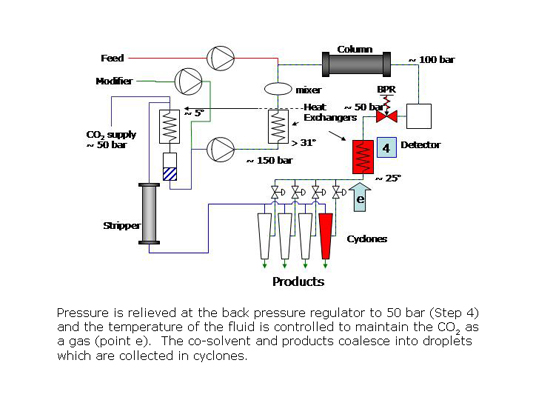
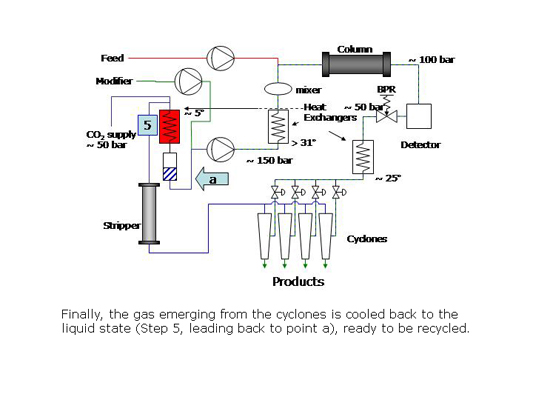
In preparative SFC, products can be easily isolated from the column effluent by reducing the pressure of the stream to bring the CO2 to the gas phase. The polar co-solvent and products condense into droplets which are recovered using gas-liquid separators while the CO2 is recycled.
Supercritical fluid video
The film clip shows the effect of heating sulfur hexafluoride while above its critical pressure. At the critical temperature the meniscus between the liquid and vapor phase vanishes as the density of the heated liquid reaches that of the pressurized gas. On cooling it reverts to the 2-phase system.